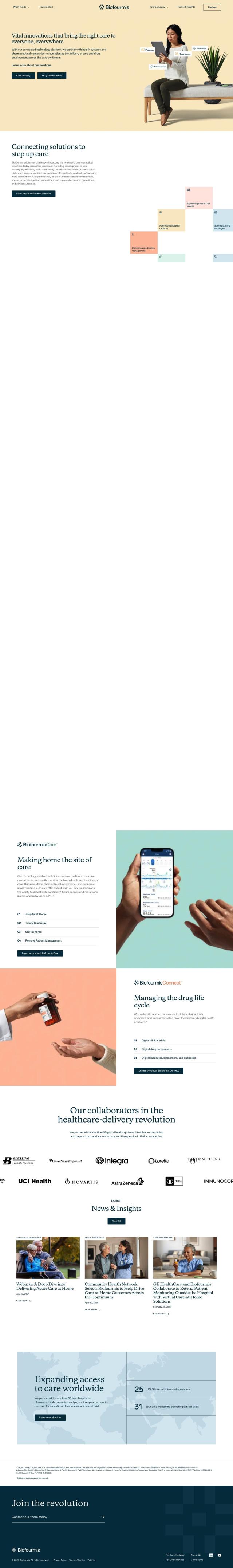
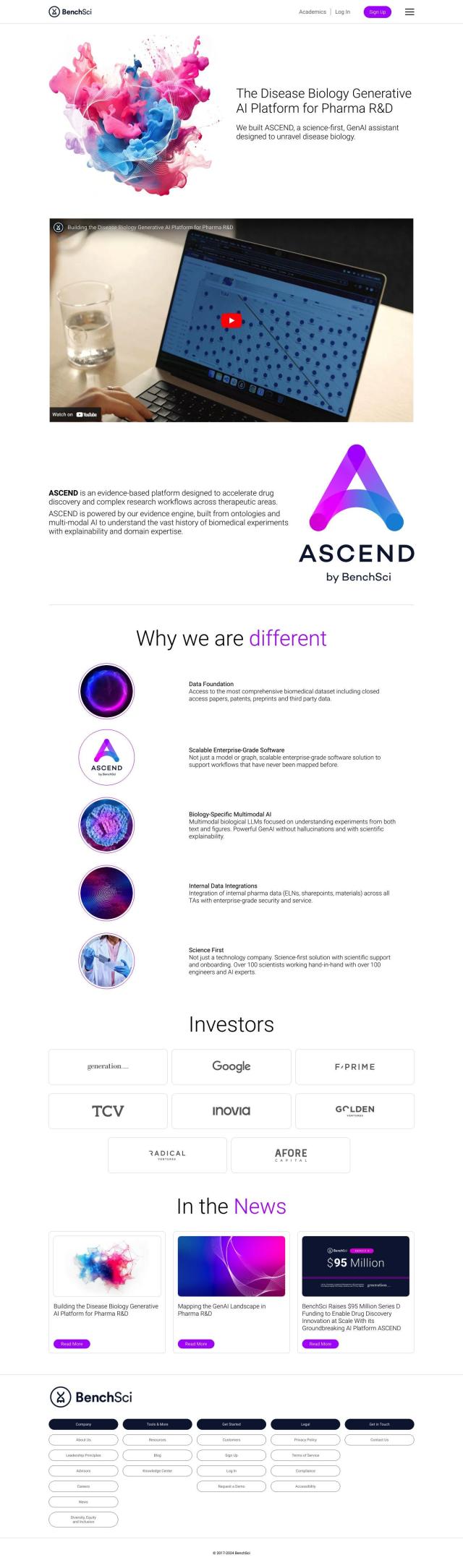
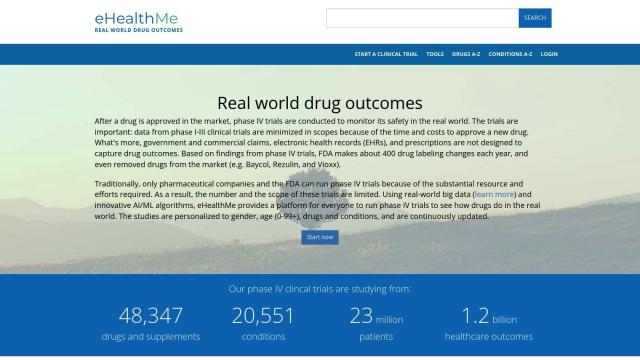
Question: Is there a system that tracks clinical trials, candidate development, and regulatory approval for pipeline intelligence?
Medidata
If you want a single system to manage clinical trials, candidate development and regulatory approval, Medidata has a big system. Medidata's product family includes Clinical Data Studio for data integration, Rave EDC for electronic data capture, Medidata AI for machine learning and other advanced analytics, and Decentralized Trials for remote participation. Medidata has 25 years of experience and has been involved in 65% of FDA-approved novel drug approvals since 2015, so its technology can help improve efficiency and patient-centricity in your clinical trials.

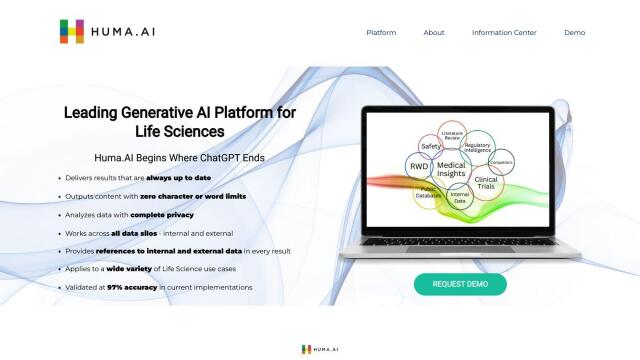
Huma.AI
Another contender is Huma.AI, which uses generative AI for medical affairs, regulatory affairs and clinical development. Huma.AI can provide detailed results without character or word limits, with complete privacy and access to internal and external data sources. It can simplify real-world data analysis and automate post-market surveillance, and it can provide results in plain language with an accuracy rate of 97%.

Verily Viewpoint
If you prefer a more integrated system, Verily Viewpoint has tools like Recruit for participant engagement, Workbench for secure analytics, Site CTMS for digitized site workflows, and Longitudinal, Participant-Centric Registries for accelerated evidence generation. The system is designed to improve research efficiency and quality by combining multimodal real-world data with prospectively collected data.
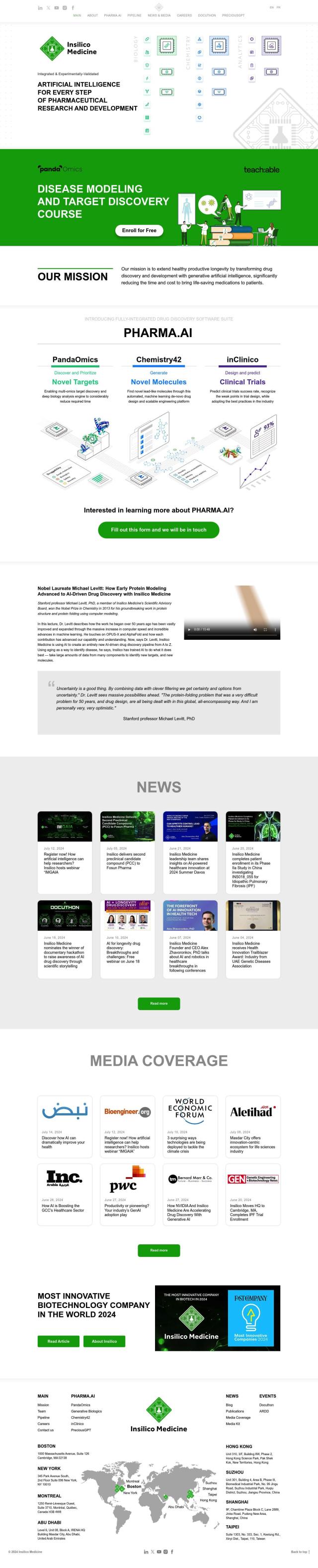

Insilico Medicine
Finally, Insilico Medicine has a suite of AI-powered tools to accelerate pharmaceutical research and development. Its platform includes PHARMA.AI for drug discovery, PandaOmics for OMICs data analysis, Chemistry42 for small molecule discovery, and inClinico for predicting clinical trial success rates. The tools can accelerate and lower the costs of traditional drug discovery methods, speeding up the development of life-saving drugs.