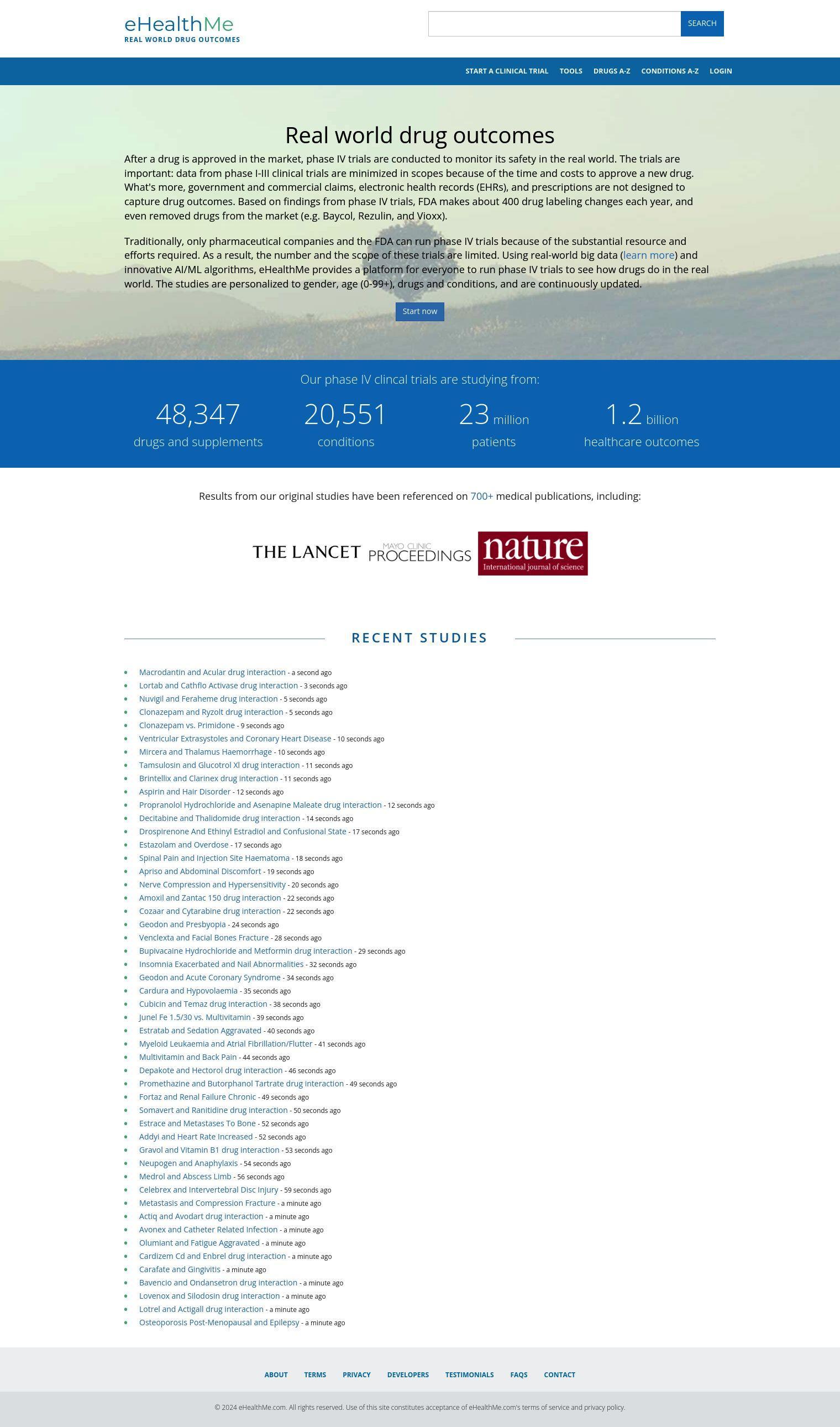
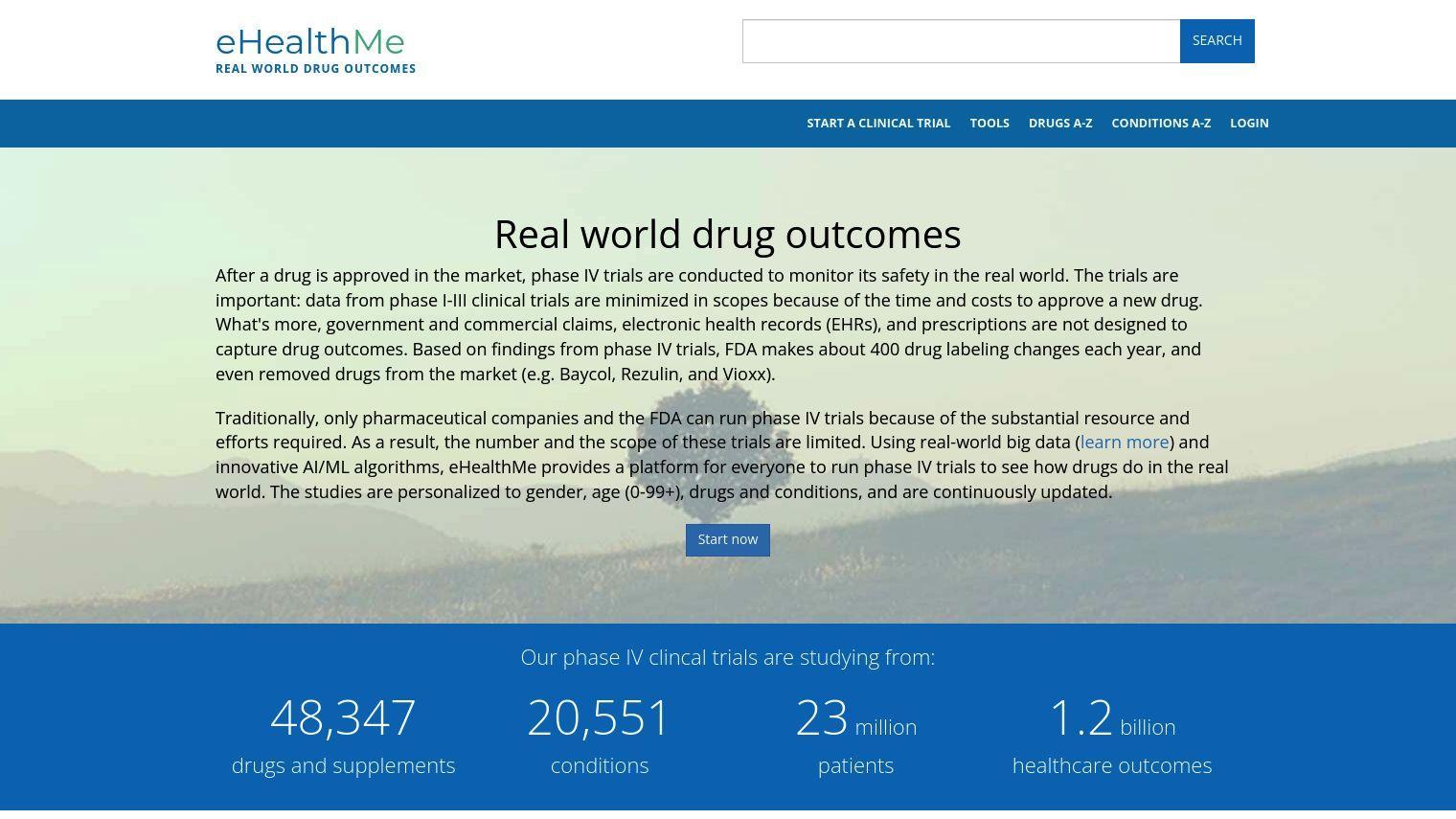
eHealthMe uses big data and AI/ML algorithms to let anyone run their own phase IV clinical trials, a process previously available only to pharmaceutical companies and the FDA. The idea is to better understand what happens to patients who take drugs in the real world, not just in the controlled conditions of phase I-III clinical trials that are limited by time and budget.
eHealthMe's studies are customized for gender, age, drugs and conditions, updated continuously and based on massive amounts of data:
- 48,347 drugs and supplements
- 20,551 conditions
- 23 million patients
- 1.2 billion health outcomes
The studies have produced more than 700 medical papers, including details on drug interactions, conditions and health effects. You can see original studies, for example, on how a particular combination of medications affects a particular health issue.
The service helps fill a gap in post-approval drug monitoring that's crucial to making informed decisions and keeping patients safe. By opening up phase IV trials to more people, eHealthMe enables more research that's broader and more detailed, which in turn should help us better understand drugs' effects and risks.
For more, check the eHealthMe website.
Published on July 16, 2024
Related Questions
Tool Suggestions
Analyzing eHealthMe...